Molar weight, also referred to as molar mass is the mass of a single molecule of a substance expressed in the unified atomic mass units. It is expressed in g/mol. As opposed to measuring, the molar mass is calculated because atoms of an element are far too small to measure manually. Calculating molar weight is as easy as a basic multiplication question. However, finding the basics like RAM can cause you to find the wrong answer. For this reason, MW Calculators were established.

What is a Molarity weight calculator?
This is an online tool that is used to calculate molar mass. To use this tool, you just need to provide the name of the element or the compound, and the tool will proceed to find the answer automatically.
How to calculate the molar weight of an element?
- The first step is to understand what the concept of molar mass or weight is all about. The molar mass is the weight of 1 mole of any substance when represented in grams. You also need to get the basic formula for calculating the molar weight. You can calculate the molar mass of any element by finding the product of the atomic mass and the factor used in the conversion of grams per mole of the substance.
- The succeeding phase would be to figure out the relative atomic mass of the element in question. The term relative atomic mass refers to the average weight of a sample and all its isotopes represented in atomic units. The relative atomic mass can be found on the periodic table of elements. Normally, it is the number that is directly below the symbol of the element. Most of the time, it is presented as a whole number with decimals.
- Now that you have the relative atomic mass, the next step is to multiply it by the constant molar mass. The constant molar mass is defined as 1 gram per mole. Thus, by finding the product of the two, you will be converting the atomic units to grams per mole.
- For samples that are only found in molecules of two or more atoms, you have to multiply the product of the procedure mentioned above by the specific number of the atoms. For instance, Hydrogen and chlorine are made up of two atoms. This means that the resulting molar mass would have to be multiplied by two.
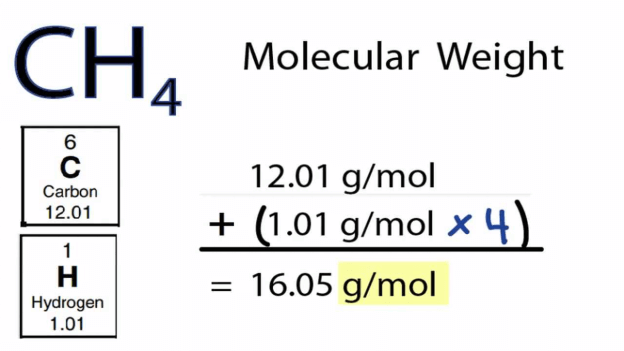
You can also calculate the molar mass of a compound or a solution. A compound is a sample that is made up of two or more elements. The fact that it is made up of more than one element means that the first step would be to determine the chemical formula of the compound. The chemical formula will help you determine how many atoms are these in each of the elements used to make the compound.
In the case of a compound, when calculating the molar weight, you must start by calculating for each of the elements. Follow the procedure explained above. Once you have the molar weight of each element in the compound, you can proceed to calculate the sum. The sum of the molar weights of each compound will give you the molar mass of the entire compound.
Conclusion
Calculating molar mass is pretty straight forward. The most important thing is to make sure you get the right relative atomic mass. The periodic table is designed to make this task effortless.